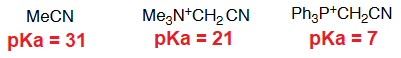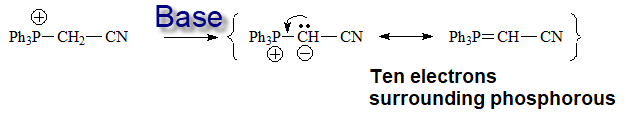ADDITION OF CARBON NUCLEOPHILES TO ALDEHYDES AND KETONES
This is a extremely important reaction because it allows us to build new single C-C or double C=C bonds and increase the complexity of the product.
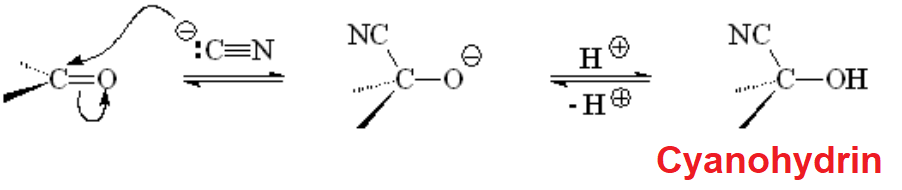
The simpler carbon nucleophile is perhaps cyanide that reacts with aldehydes and ketones to render cyanhydrins.
The reaction is very simple and useful because the CN group can be converted in many other functional groups.
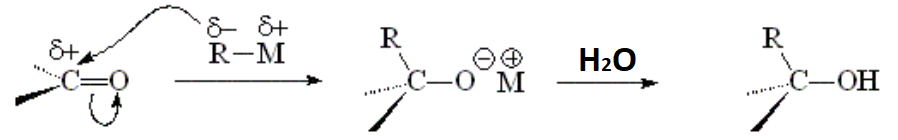
LITHIUM AND MAGNESIUM ORGANOMETALLICS (GRIGNARD)
Depending on our use of formaldehyde, an aldehyde or a ketone, the outcome will be a primary, secondary or tertiary alcohol, respectively.
IMPORTANT: There are numerous functions that may compete or simply destroy the organometallic, like alcohols, amines and carboxylic acids amidst many others.
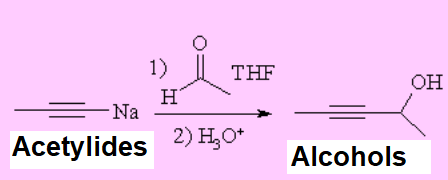
Alkynylides or acetylides, produced from the action of a strong base over a terminal alkyne, are excellent nucleophiles that easily add to carbonyl groups.
IMPORTANT: There are numerous functions that may compete or simply destroy the alkynylide, like alcohols, amines and carboxylic acids amidst many others.
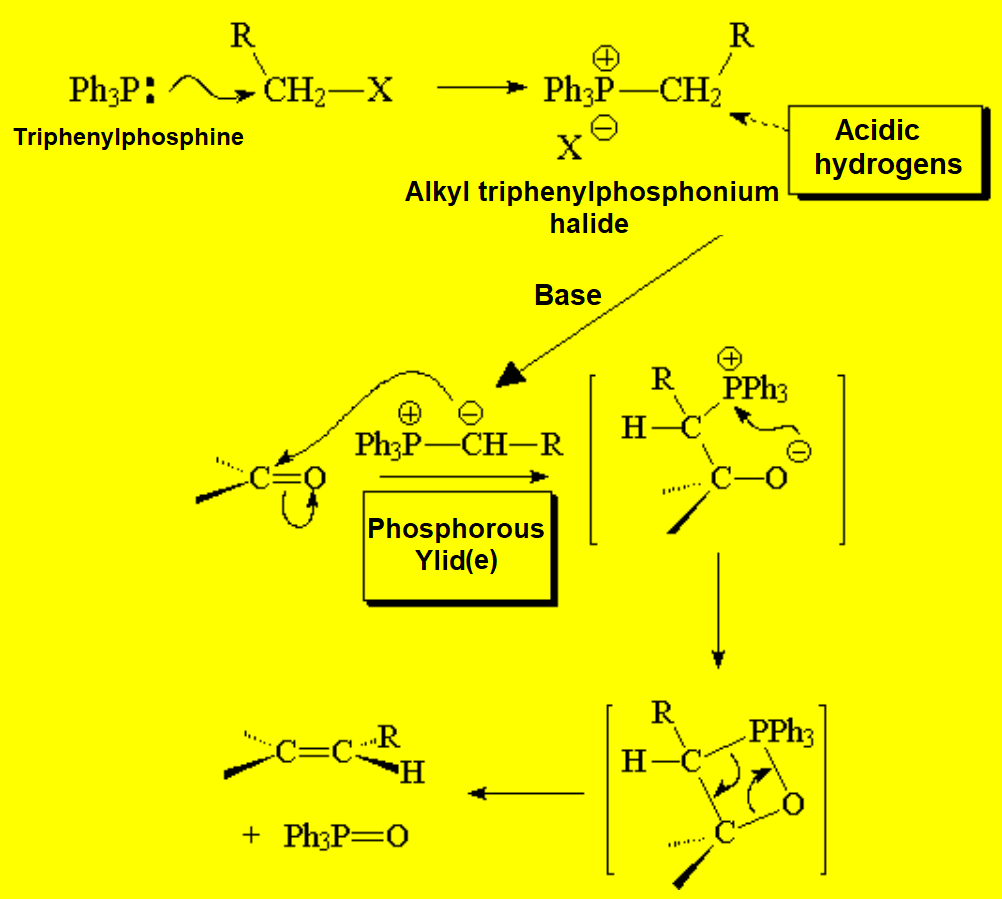
This reaction was already mentioned in the chapter of alkenes because it is used for their preparation.
The key compound in this process is the phosphorous ylid(e), that is formed due to the unexpected acidity of the hydrogens neighboring a phosphonium salt.
This is an important property that the analogous ammonium salts do not bear.
What is the reason for the unexpected acidity?
The only way to explain the acidity of a phosphonium salt is that phosphorous can put into play its 3d orbitals in order to delocalize the negative charge on the carbon of the formed ylid(e).
Nitrogen cannot!!!