You already saw what a multiplet is.
When a group of equivalent 1H shows up as a “N-plet” is because there are “N-1” vicinal 1H's.
Do you understand it?
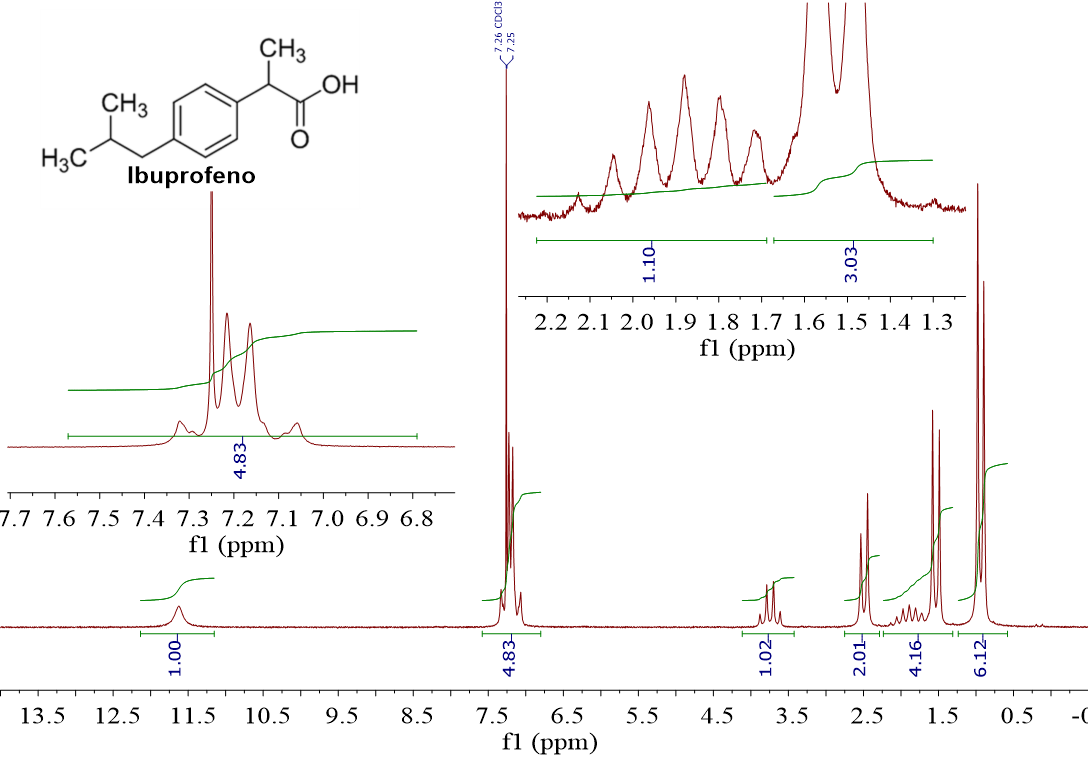
I just kind of cheated you because we've seen that in the opposite direction: a group of vicinal 1H's to another group of “N” 1H's makes the latter be a “N+1-plet”. Ibuprofen is an excellent example:
The most "shielded" signal (0.9 ppm) integrates for 6 and is a “doublet”, i.e. it is the case of 6 equivalent 1H's vecinal to a single one. In ibuprofen's structure, the two CH3 groups on its left hand side comply with these conditions.
The following signal (1.5 ppm) integrates for 3 (look at the inset expansion) and is a “doublet”. In other words, the signal belongs to 3 equivalent 1H's vecinal to a single one. In ibuprofen's structure, the upper CH3 group matches with all this.
The next signal (1.5-2.1 ppm) is quite complex and is partially "overlapped" with the doublet at 1.5 ppm. We'll take a look at it later on.
Next, the signal at 2.5 ppm integrates for 2 and is a "doublet", i.e, 2 equivalent 1H's vicinal to a single one. This signal must be assigned to the only CH2 group in the molecule, vicinal to a CH group.
At 3.7 ppm there's one signal that integrates for 1 1H and is a "quartet" and so, it belongs to a single 1H vicinal to 3 1H's. This is only complied by the upper CH group in ibuprofen.
At ca. 7.2 ppm there's a complex signal whose integral is almost 5.
The quite deshielded chemical shift strongly suggests aromatic protons but ibuprofen only bears 4 of them.
Be careful!
In the expansion one can easily see that there is a thin signal at 7.26 that corresponds to the solvent CDCl3. Its contribution to the integral is misleading.
The shape or multiplicity of the real signals at 7.2 ppm belonging to ibuprofen is complex because the ring symmetry makes the 4 hydrogens be equivalent in pairs, each pair bearing a very similar chemical shift (7.10 ppm y 7.25 ppm, aproximadamente).
The last singlet signal has a very high chemical shift, a extremely deshielded one (11.6 ppm).
The 1H's of the COOH and the CH group in the left hand side of ibuprofen remain to be assigned. The COOH does not have neighboring 1H's and therefore this should be it.
How many neighbors does the remaining CH group have?
Count 6 1H's of the CH3 groups plus 2 of the CH2 group: a total of 8 1H's!!! That makes 8+1 “legs”. Unfortunately not all of them can be seen because of the overlapping with the doublet at 1.5 ppm.
Why do the 1H's with neighboring 1H's split and look like multiplets?
The answers are in the following links...