The alkanes are the least reactive organic compounds because they lack functional groups and, as a consequence, of polarized bonds.
Paradoxicaly, bond energies of alkanes are not very different from those of other compounds which we do know are much more reactive:
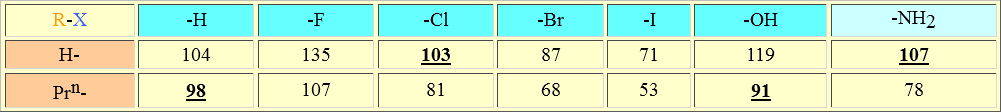
Similar energies are required to break a C-H bond in propane (98 kcal/mol), a Cl-H bond in hydrogen chloride (103 kcal/mol), a C-O bond in propanol (91 kcal/mol) or a N-H bond in ammonia (107 kcal/mol). But we know that any of the last three molecules is much more reactive that propane. How come?
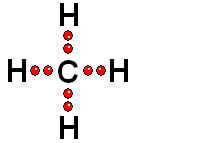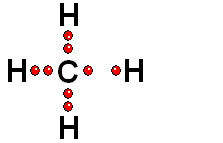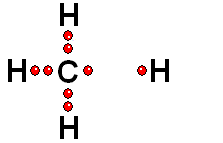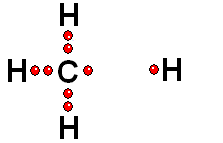 Two species with an odd electron each are produced. They are called FREE RADICALS.
Two species with an odd electron each are produced. They are called FREE RADICALS.
HOMOLYTIC cleavage:
The two bond electrons are split between the two bonded atoms. This way of breaking a bond is in general very difficult and only happens when the hydrogen is attached to an atom of low electronegativity.
In general, alkanes are only able to react through HOMOLYTIC cleavage, which is quite energy costly.
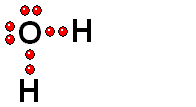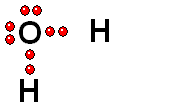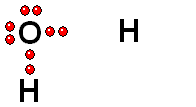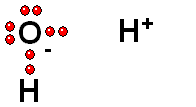 Two charged species are formed: an ANION (negative) and a CATION (positive).
Two charged species are formed: an ANION (negative) and a CATION (positive).
HETEROLYTIC cleavage:
The two electrons are kept by the atom of the highest electronegativity. This is the most common way of breaking a bond and happens when the hydrogen is attached to an atom of high electronegativity.
TYPICAL REACTIONS OF ALKANES
 Two species with an odd electron each are produced. They are called FREE RADICALS.
Two species with an odd electron each are produced. They are called FREE RADICALS.
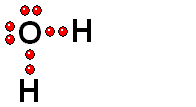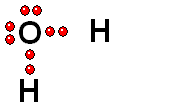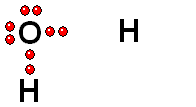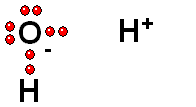 Two charged species are formed: an ANION (negative) and a CATION (positive).
Two charged species are formed: an ANION (negative) and a CATION (positive).