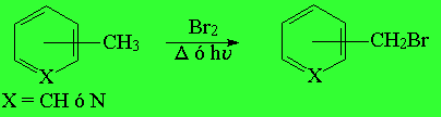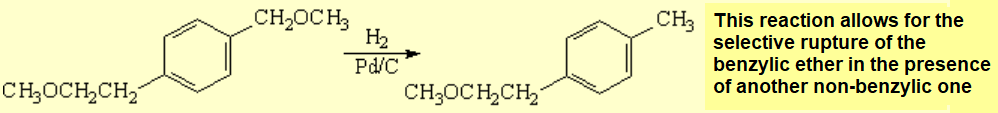THE BENZYLIC POSITION:
ALKYLARENES
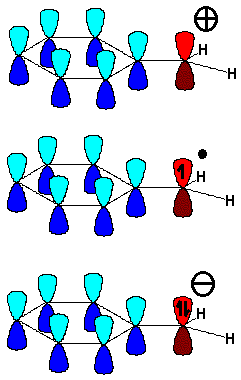
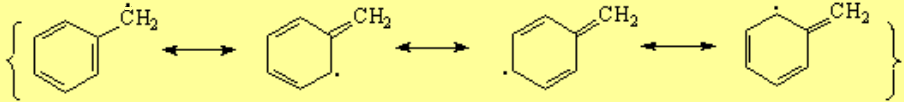
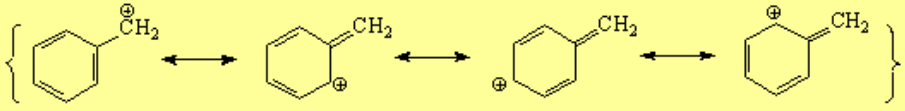
It is fairly easy to induce electron excess or deficiency at the benzylic position because the aromatic "pi" cloud stabilizes it.
The carbon bonded to the aromatic ring, regardless it is a carbocation, radical or carbanion, adopts a sp2 hybridization and its "remaining p orbital" conjugates with the ring "pi" orbitals.
Reactions going through a benzylic carbocation, radical or carbanion are easier than expected.
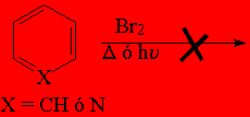
The aromatic compounds like benzene or pyridine DO NOT REACT with bromine without catalyst as alkenes (addition) or alkanes (radical halogenation) do.
Toluene and any of the three possible methylpyridines DO REACT with bromine without catalyst but at their methyl group (radical halogenation) and with special easiness.
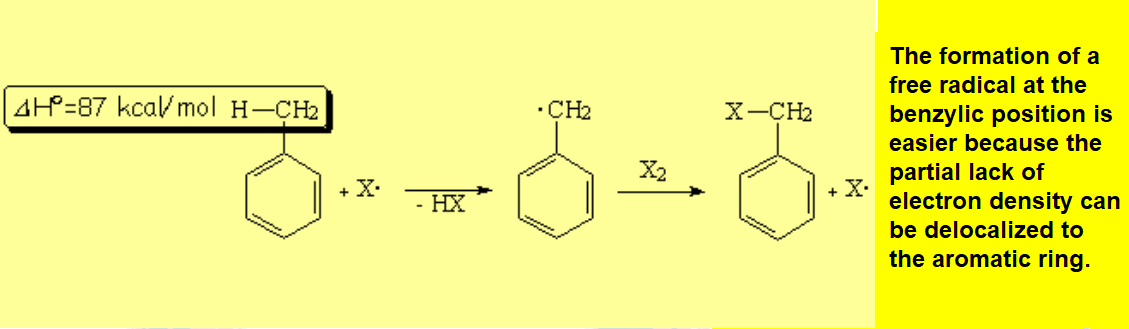
Benzylic halogenation is specially favorable. It costs less energy than at a "normal" alkyl carbon. The C-H bonds at the benzylic position are slightly weaker than expected.
NUCLEOPHILIC SUBSTITUTION
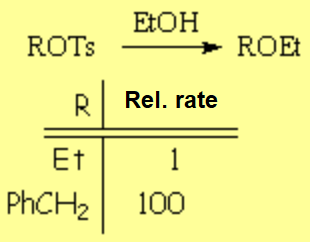
The solvolysis of a tosylate is faster at the benzylic position because it probably goes through a benzylic carbocation, less unstable than a "normal" alkyl carbocation.
Click here to recollect the aliphatic SN.
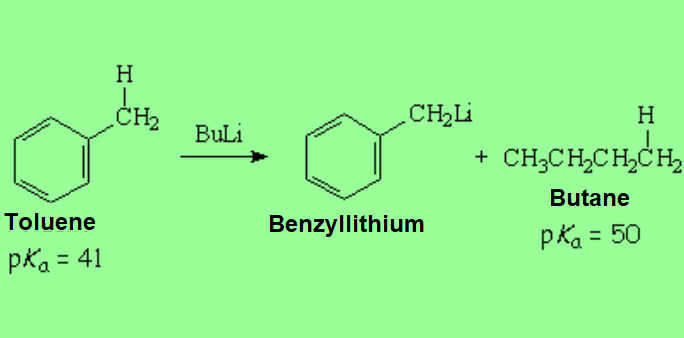
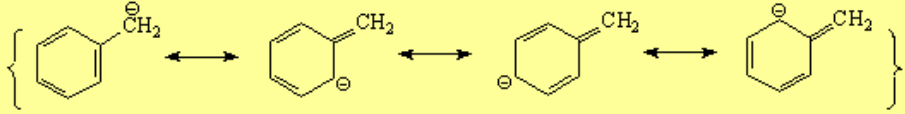
Toluene can loose a proton from its methyl group that apparently is a normal sp3 one.
That can be done with a strong base like butyllithium whose carbons are sp3 as well.
However, toluene's pKa is 9 orders of magnitude lower than butane's (toluene is 1.000.000.000 more acidic than butane) because the benzylic carbanion is stabilized by resonance with the aromatic ring. That's why it is obtained with less difficulty than expected.
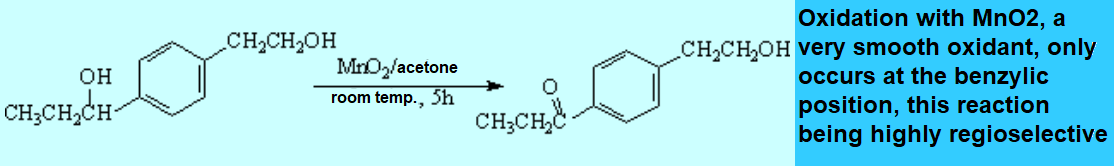
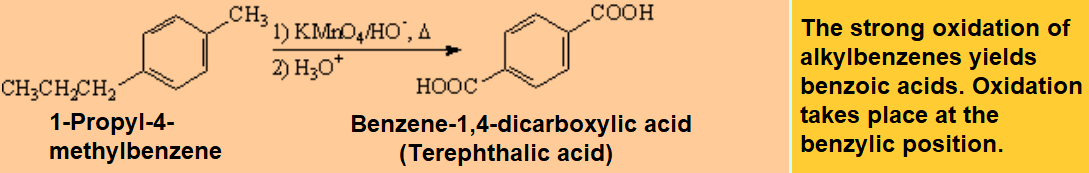
Oxidation processes usually get through the formation of free radicals. That's why they are prone to be effected at benzylic positions.
The selective hydrogenolysis of groups bonded to the benzylic position can be easily achieved like for instance that of an ether.