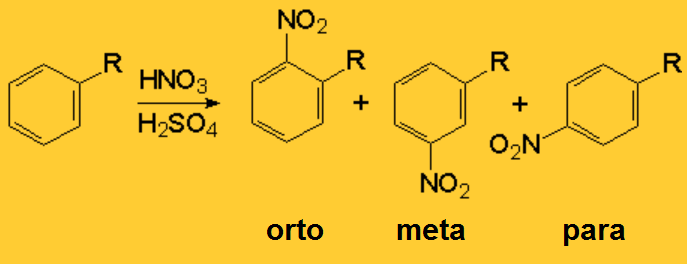
We can perform a SEAr over an aromatic compound already bearing substituents, but we have to wonder a couple of questions:
Would the present substituent make the SEAr easier or more difficult?
Would the present substituent direct the incoming group to a given position?
The following table contains the experimental reaction rates (krel) relative to benzene (R=H) and the "ortho, meta and para" regioisomer ratio that is obtained in the nitration of various monosubstituted benzenes.
What conclusions do you arrive to?
The redish color of the rings is a qualitative indication of their electronic density: the more redish, the higher.
Click on the different figures to get an explanation.
One can distinguish two kinds of groups regarding the reaction rates relative to benzene:
Those ones diminishing it:
They are electronegative groups that remove electron density from the ring thus making the SEAr more difficult.
Those ones increasing it:
They are electron donors that increase the ring electron density and facilitate the SEAr.
One can distinguish two kinds of groups depending on the orientation they induce:
meta directors
:
They are coincident with strongly electronegative, deactivator groups.
ortho-para directors
:
They could by either electron donors and activators, like OH and CH3, or slightly electronegative and soft deactivators like CH2Cl, Cl and Br.