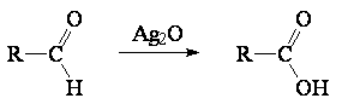PREPARATION OF CARBOXYLIC ACIDS BY OXIDATION
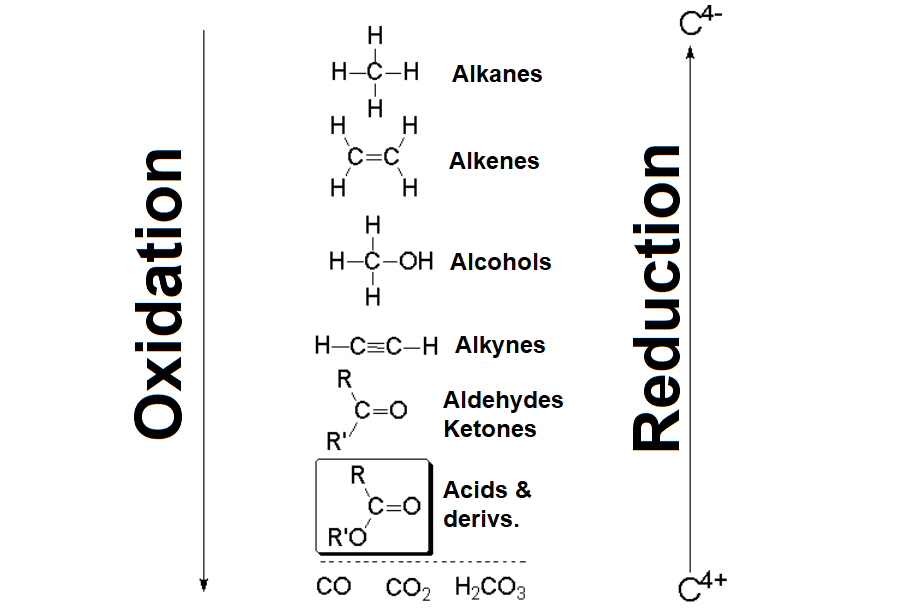
The carboxyl carbon of carboxylic acids and their derivatives is in +3 formal oxidation state, the highest possible in the ranking of organic functional groups.
Carboxylic acids could be thereupon obtained by oxidation of almost any other functional group, provided there exists the appropriate oxidant and the right reaction pathway.
Alkanes are difficult to be selectively oxidized. Nevertheless, the benzylic position is easily oxidized.
The strong oxidation of alkylbenzenes renders benzoic acids. Oxidation thus occurs at the benzylic position.
Alkenes and Alkynes Oxidation
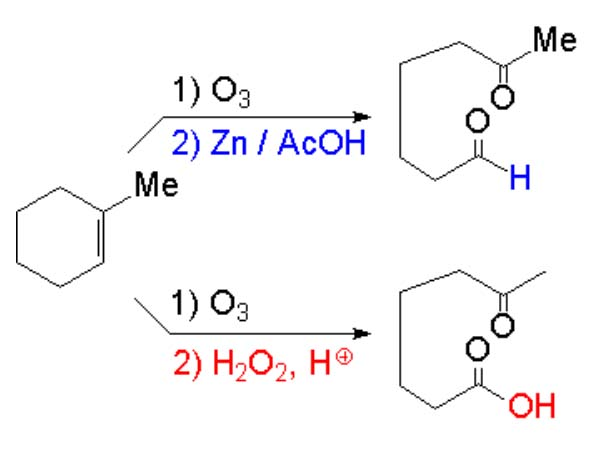
Alkenes can render carboxylic acids by oxidation but suffering double C=C bond cleavage.
Alkynes as well but the reaction is not usually useful enough.
Primary alcohols are the only ones that can be oxidized to carboxylic acids without any C-C bond breakage.
Secondary and tertiary alcohols can be oxidized under very stringent conditions but undergoing C-C breakage, actually limiting the usefulness of this reaction.
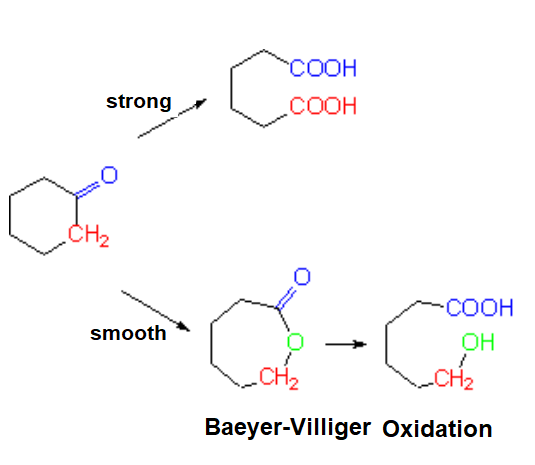
Ketone oxidation must go through C-C bond cleavage.
If strong (KMnO4, K2Cr2O7), two carboxylic acids are obtained.
If smooth (Baeyer-Villiger oxidation), an ester is produced that can be hydrolized to render an alcohol and a carboxylic acid.
Click here to look back on ketone oxidation.