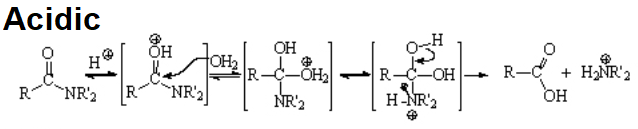
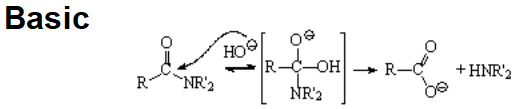
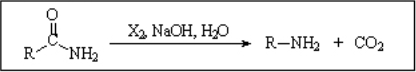
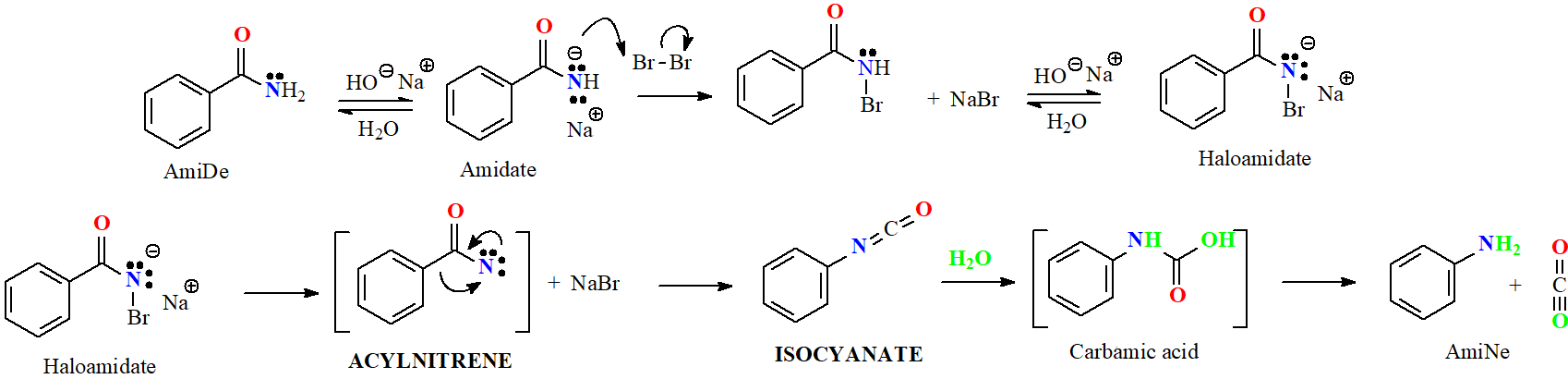
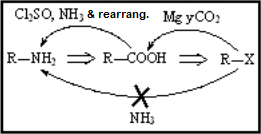
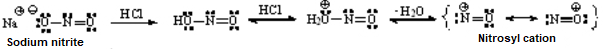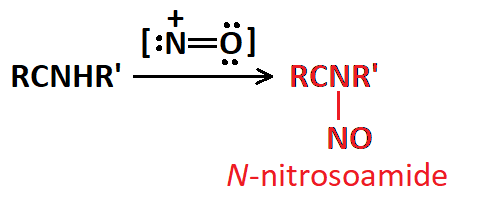Amides are the least reactive among all carboxylic acid derivatives because the electrophilicity of the C=O group is heavily reduced by the electron-donor nitrogen. Despite that, there are some interesting reactions...
The hydrolysis of an amide is usually difficult a process that requires strong acids or bases and high temperatures.
IMPORTANT: If there are other functions present, they wouldn't endure the reaction.
As it is customary, the addition-elimination mechanism applies.