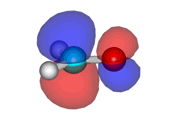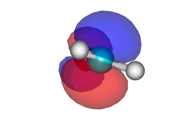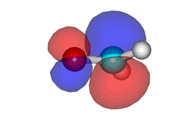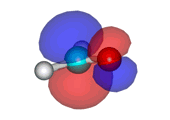
 Formaldehyde's LUMO
Formaldehyde's LUMO
Lowest Unoccupied Molecular Orbital (LUMO) of formaldehyde, ready to accept electrons from the Highest Occupied Molecular Orbital (HOMO) of an electron-rich species. The attack will take place on carbon which bears the orbital highest coefficient.
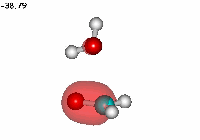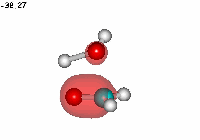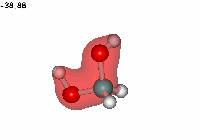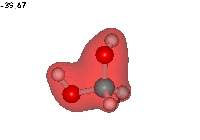 Water addition to formaldehyde
Water addition to formaldehyde
Formaldehyde's carbon, initially planar with sp2 hybridization, suffers the attack of the electron cloud of water's oxygen. The reaction proceeds through a TS where the carbon turns into a tetrahedron, swapping its hybridization from sp2 to sp3.
 Formaldehyde's LUMO
Formaldehyde's LUMO
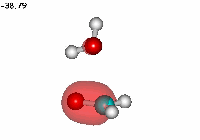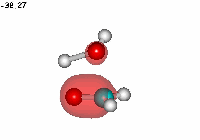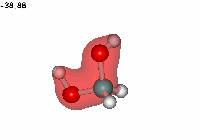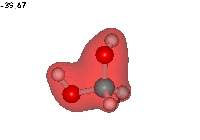 Water addition to formaldehyde
Water addition to formaldehyde