NUCLEAR MAGNETIC RESONANCE (NMR)
Rabi, Bloch y Purcell observed that certain atomic nuclei like the proton (1H) and phosphorous (31P) absorbed radiofrecuency (RF) when they were immersed in a magnetic field. The radiofrecuency was different for each nucleus and that allowed for their identification. At the very moment the nuclei absorbed the RF, it is said that "resonance" is attained.
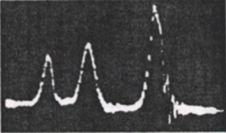 One of the first 1H-NMR recorded spectra (circa 1950, pure ethanol).
One of the first 1H-NMR recorded spectra (circa 1950, pure ethanol).
Chemistry is understood as matter transformations due to electron racking between atoms or molecules.
In Chemistry, atomic nuclei pass by unconspicuous because they play a secondary, though important, role in the electron racking.
But let's not forget that there is a nucleus at the very center of every atom and, although tiny in size, the nuclei bear properties we can use to visualize the molecular stucture.
One of those properties is the magnetic moment which is precisely exploited in Nuclear Magnetic Resonance (NMR).
The first physics experiments, foundation of NMR, were performed in 1938 by Isidor Rabi, Nobel Prized six years later for that. Other important physicists in the discovery of NMR were Felix Bloch and Edward Mills that extended the observation of atomic magnetic properties to liquids and solids. They shared the Nobel Prize in 1952.
That very same year, the company VARIAN patented and launched into the chemistry market the first commercial NMR spectrometer named HR-30.
As the physics experiments got more sophisticated, chemists realized that observing one nucleus, for instance 1H, molecules rendered different RF signals corresponding to the different types of hydrogen.
As early as in 1952, it was observed that ethanol (CH3CH2OH) rendered three signals in its 1H-NMR spectrum.
They were assigned to the three CH3 hydrogens on one hand, the two CH2 hydrogens on the other, and the third signal to the OH hydrogen.
Chemists were nicely surprised not only by that finding but by the fact that the areas occupied by the three signals followed the ratio 3:2:1, exactly the number of hydrogens responsible for each signal.
The scientific community very rapidly got conscious of the importance of the discovery: NMR was able to give a signal for each type of hydrogen in a molecule and its position on the RF scale was an indication of their place in the molecular structure.
It had to be the electrons, acting now as supporting actors, the ones responsible for the nuclei 'resonating' at slightly different values of RF.
The coincident timing between two discoveries in the 50's, NMR and the transistor, has twined their progress and NMR has strongly benefitted from electronics improvement and miniaturization, reaching nowadays spectacular performances, not only in the Chemistry field but in Biochemistry, Biology and Medicine.
 One of the first 1H-NMR recorded spectra (circa 1950, pure ethanol).
One of the first 1H-NMR recorded spectra (circa 1950, pure ethanol).
